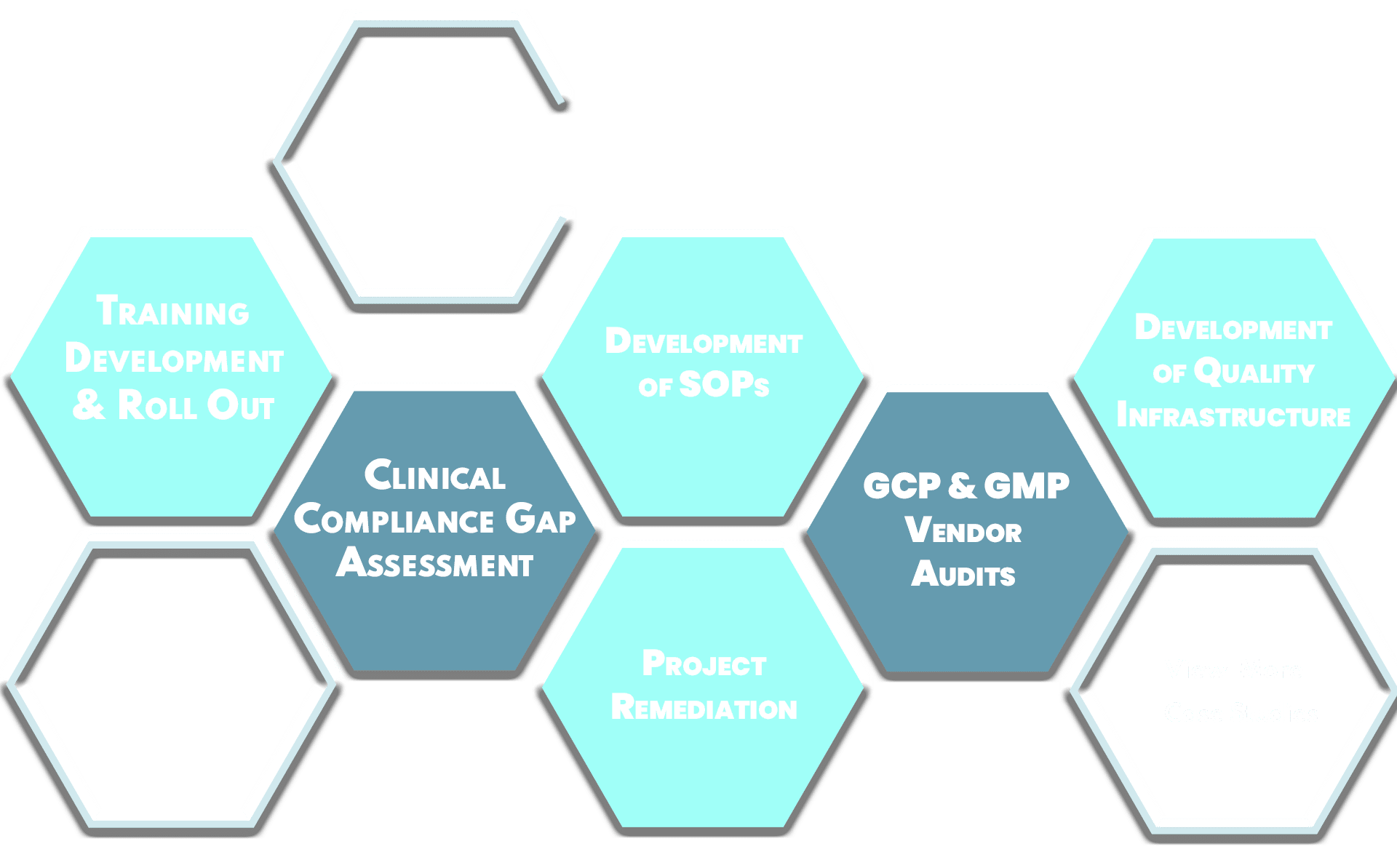
About DArcy
DArcy Compliance Consulting was formed to address a very specific need in the Life Science marketplace – the need for a practical and implementable quality infrastructure in the early stages of a company’s product development journey. We work with companies who are conducting laboratory experiments and need to instill robustness in their processes, or are starting clinical trials and need to align with ICH guidelines or may be preparing for a Regulatory Inspection. DArcy Compliance Consulting wants to be your partner for building compliance and quality in your organization.
DArcy Compliance Consulting
Specializes in Life Science Small Companies
and Start-Ups
Testimonials
Start-Up Client – Gene Therapy
“The team at Darcy Compliance Consulting have been an invaluable resource in establishing a compliance framework that meets the needs of Regulatory agencies. Their pragmatic approach is perfect for Start-Up biotechs looking for a fit-for-purpose solution that is scalable but not overly burdensome for the organization.”
- Head of R&D Operations
Start-Up Client – Gene Therapy
“Having a seasoned team with broad industry experience was critical for us since many of the legacy SOPs came from academia and needed to be upgraded to meet industry standards. It was a great pleasure to work with a team so adept at partnering with small companies.”
- Head of R&D
Start-Up Client – Biotech
“The team at DArcy Compliance Consulting were sensitive to the limited bandwidth of functional staff and helped the team prioritize the critical areas to address. I recommend them wholeheartedly.”
- Head of R&D Operations
Pharma Client
“DArcy provided excellent structure to the project to understand what regulatory changes were required and how to implement. They were very strong in laying the foundation for the project, assigning accountabilities and ensuring deliverables were met. It has been a pleasure working with them.”
- Senior Director
Start-Up Client – Biotech
“The team at DArcy demands excellence and delivers on it in their preparation and drafting of procedural documents and associated training.”
- Compliance Business Partner
Start-Up Client – Biotech
“Experts, like the team at DArcy, navigate our commercial and medical stakeholders to execute their activities with integrity and confidence in what is a highly regulated industry. We are all the wiser for their commitment to simple, but impactful compliance guidance.”
- Compliance Executive
Pharma Company
“To the DArcy team, very many thanks for your leadership of this project. I’ll say now, an ambitious project that required continual drive and exceptional customer/supplier engagement. You did a great job on all fronts. I’m extremely pleased with the outcome.”
-Senior Director - Pharma Company
Pharma Company
“I asked DArcy to oversee a restructuring of two of my departments. The team did an outstanding job, leading by example and overcoming resistance by relentless focus on the benefits. They were right on target with their assessment and solutions developed.”
- VP Clinical Pharma Company
Start-Up Company – Gene Therapy
“Working with DArcy Compliance Consulting over the past 3 ½ years has been one of the chief highlights of my time here. Your commitment to excellence, and your incredible competencies, and dedication are all traits I will not ever forget.”
- Compliance Business Partner
Start-Up Company – Small molecules
“As a small Start-Up, we struggle with the bandwidth to independently implement all facets of the quality infrastructure needed. Collaborating with the DArcy consultant team has been a valuable resource for us. We appreciate them sharing their expertise and the insight they’ve gained from working with other small companies like ours.”
- VP Quality & Compliance
Start-Up Company – Chronic Neurological Treatments
“Their gap assessment, and the SOPs they wrote, have been instrumental in moving us in the right direction. We will definitely work with them in the future and would highly recommend the DArcy Team to others with quality consulting needs.”